
Home / News
-
-

-
Bio-Techne said on Wednesday that it has recently received CE marking and launched in Europe its assay for human papillomavirus for use in patients with head and neck cancer.2022-07-07View More
-
-
-

-
Recently, Wuhan YZY Biopharma Co., Ltd completed a 200 million CNY Pre-C round of financing. This round of financing was completed by the joint investment of Wuhan Hi-Tech, Hubei Science Technology Investment and Optics Valley Financial Holding Group.2022-07-06View More
-
-
-
-
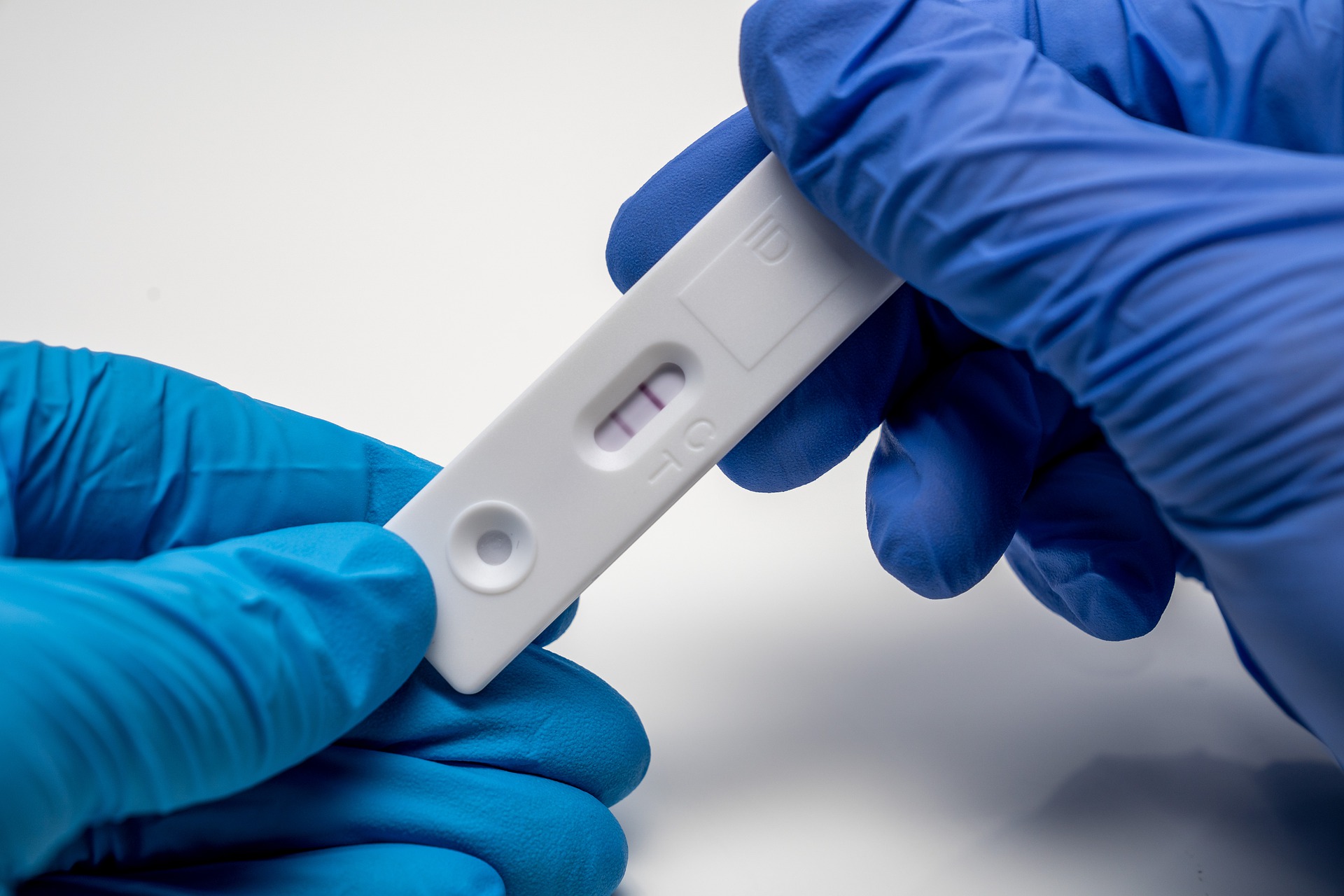
The FDA cautioned in late 2021 after the omicron variant emerged that antigen tests may have reduced sensitivity, meaning they correctly identify fewer people with COVID-19. Data have continued to accumulate that the tests are not as sensitive to the newer variants of the virus, Stenzel said.2022-07-06View More
-
-
-

-
Forgetfulness and memory loss can be a normal part of aging, but can also signify the onset of dementia, one of the major causes of disability and dependency in old age.2022-07-06View More
-
-
-
-
Although Covid-19 is not over yet, the market conditions of nucleic acid and antigen detection reagents are rapidly cooling down, and even "cliff-like" subsidence.2022-07-05View More
-
-
-
-
Despite significant and stunning advances in vaccine technology, the COVID-19 global pandemic is not over. A key challenge in limiting the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is identifying infected individuals.2022-07-05View More
-
-
-

-
New research led by Edith Cowan University has made an important discovery that could lead to more effective treatments for the world’s 262 million asthma sufferers2022-07-05View More
-
-
-

-
On July 2, 2022, Mgi Tech Co., Ltd and Guangzhou Burning Rock Biotech Co., Ltd. officially reached a strategic cooperation.2022-07-04View More
-
-
-
-
FDA issued a recommendation on June 30, 2022 to COVID-19 vaccine manufacturers that indicated that they should include an Omicron subvariant BA.4/5 component in their vaccines.2022-07-04View More
-
-
-

-
PreventionGenetics said Thursday that it has been granted marketing authorization for its POMC/PCSK1/LEPR companion diagnostic as a Class II device by the US Food and Drug Administration.2022-07-04View More
-
-
-

-
Recently, the China Chamber of Commerce for Import and Export of Medicines and Health Products (CCCMHPIE) announced the second batch of Certificate of Export Commodity Brand list recommended by the chamber of Commerce for medical insurance in 2022.2022-07-01View More
-
-
-

-
Becton Dickinson has obtained the CE-IVD mark on a combined panel assay to detect SARS-CoV-2, influenza A and B, and respiratory syncytial virus. The assay runs on the firm's BD Max instrument and is now available in countries that recognize the CE mark, the firm said on Thursday.2022-07-01View More
-
-
-

-
Visby Medical said on Wednesday that it has added $35 million to its Series E financing round, bringing its total proceeds from the round to more than $135 million.2022-07-01View More
-
-
-

-
On June 28, the National Health Commission of the PRC released the "COVID-19 Prevention and Control Plan (Ninth Edition)". Compared with the eighth edition, the following revisions were made:2022-06-30View More
-
-
-

-
Roche announced on Tuesday that it has received CE marking for its Ventana DP 600 slide scanner for digital pathology.2022-06-30View More
-
-
-

-
Anitoa Systems announced on Wednesday that its PCR-based test for monkeypox has received CE marking.2022-06-30View More
-
-
-

-
As the grass-roots organization of CAST, Sansure Biotech attaches great importance to the construction of the enterprise association for science and technology, gives full play to the advantages of scientific and technological talents, innovation platforms, and good basic conditions.2022-06-29View More
-
-
-

-
Illumina said on Tuesday that it has invested an undisclosed amount in Time Boost Capital, a genomics venture fund that plans to match funding raised by startups participating in the UK-based version of Illumina's accelerator program.2022-06-29View More
-
-
-

-
Memorial Hermann Health System to launch a new population health program targeting 100,000 individuals.2022-06-29View More
-
-
-

-
Since Beckman Coulter began to develop in China more than 40 years ago, it has continued to increase local investment, firmly helped China's laboratory medicine, realized the localization of a number of products, and is committed to promoting leading science and technology to more people.2022-06-28View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

