
Home / News
-
-

-
Quidel on Thursday said that it expects its first quarter revenues to nearly triple year over year, primarily on sales of COVID-19 testing products.2022-04-11View More
-
-
-

-
The scientists, led by Joseph Vinetz, MD, an infectious diseases specialist, were interested to find out if an oral medication used to treat pancreatitis could reduce the viral load (the amount of virus in your body) of SARS-CoV-2 and improve symptoms in people newly diagnosed with COVID-19.2022-04-11View More
-
-
-

-
The Biden-Harris Administration announced on April 4 that more than 59 million Americans with Medicare Part B, including those enrolled in a Medicare Advantage plan, now have access to Food and Drug Administration (FDA) approved, authorized, or cleared OTC COVID-19 tests at no cost.2022-04-08View More
-
-
-

-
SeekIn said on Thursday that it has received CE marking for its LeukoPrint Molecular Karyotyping Kit for the diagnosis and stratification of leukemia patients.2022-04-08View More
-
-
-

-
The body¡¯s ability to respond to various types of stress is essential for maintaining health, and failure of such adaptive stress responses can trigger or worsen numerous diseases.2022-04-08View More
-
-
-
-
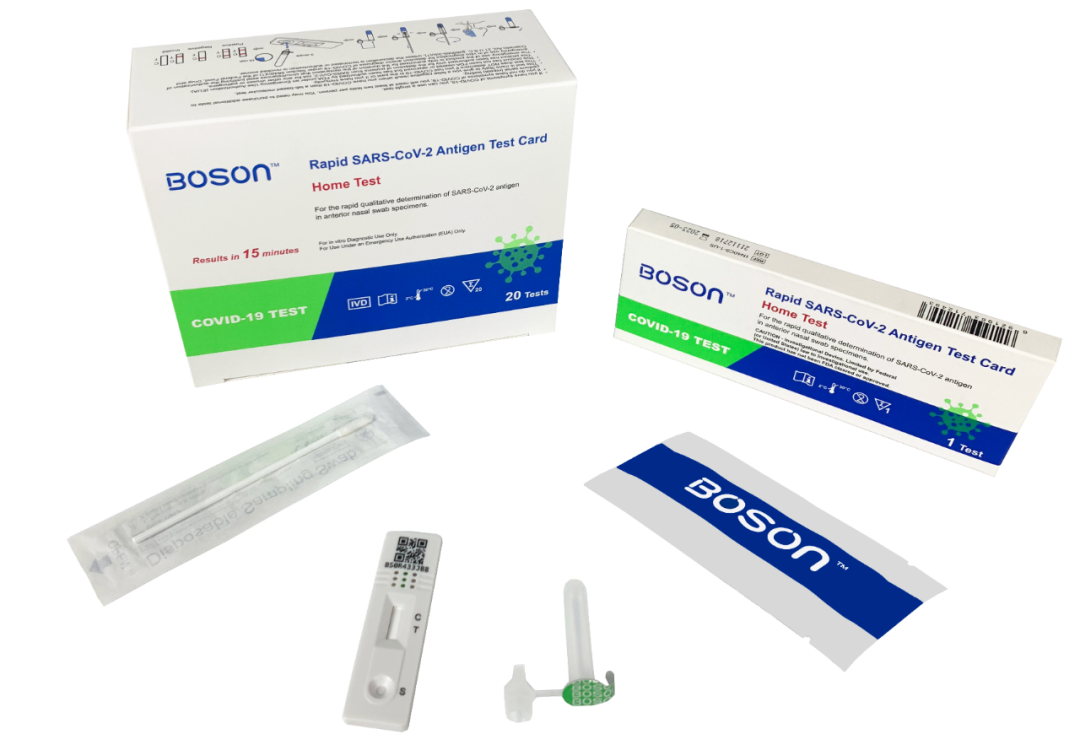
On April 6, it was reported that the Rapid SARS-CoV-2 Antigen Test Card produced by Xiamen Boson Biotech Co., Ltd. has obtained the FDA Emergency Use Authorization (EUA).2022-04-07View More
-
-
-

-
Seegene said on Wednesday that it has received Australian approval and CE-IVD marking for its Allplex RV Master Assay for respiratory viruses.2022-04-07View More
-
-
-

-
April 5, 2022 ¨C GE Healthcare and Elekta (EKTA-B.ST) announced today that they have signed a global commercial collaboration agreement in the field of radiation oncology.2022-04-07View More
-
-
-

-
On 28 March, Sanxing Medical Electric Co., Ltd. (601566.SH), made several announcements announcing that its subsidiary, Rehabilitation Investment, intended to acquire 100% of the equity interests in five hospitals for a total price of CNY 844 million.2022-04-06View More
-
-
-

-
UK life science tools company LGC said on Friday that it has acquired Rapid Genomics, a next-generation sequencing genotyping company based in Gainesville, Florida.2022-04-06View More
-
-
-

-
Agilent Technologies on Tuesday announced it has obtained expanded CE-IVD marking for the use of its PD-L1 IHC 28-8 pharmDx immunohistochemical assay as a companion diagnostic test to guide treatment for newly approved indications for Bristol Myers Squibb's Opdivo.2022-04-06View More
-
-
-

-
Jinan Babio Biotechnology Co., Ltd. recently received an official notification from the EU Notified Body for its self-tested novel coronavirus antigen detection product, and subsequently obtained the CE certification issued by PCBC.2022-04-02View More
-
-
-

-
Thermo Fisher Scientific¡¯s new Gibco CTS Xenon Electroporation System aims to provide easier scale up for cell therapies, from clinical development to commercial manufacturing.2022-04-02View More
-
-
-

-
Israeli diagnostics firm Todos Medical on Friday reported a nearly 13 percent year-over-year increase in revenues for the fourth quarter on increased demand for its COVID-19 testing products and services, as well as nutritional supplements.2022-04-02View More
-
-
-

-
On March 30, China National Medical Device Co., Ltd. (CNMD for short) under Sinopharm Group and Suzhou Hybiome Biomedical Engineering Co. Ltd., subsidiary of BioMerieux in China held a signing ceremony of the strategic cooperation agreement.2022-04-01View More
-
-
-

-
HTG Molecular Diagnostics reported after the close of the market on Tuesday that its 2021 revenues edged up 5 percent to $8.9 million from $8.5 million a year ago.2022-04-01View More
-
-
-
-
A recent study published in Nature Genetics identified 10 new genetic regions associated with Brugada syndrome, a cardiac arrhythmia disorder associated with sudden death in young adults.2022-04-01View More
-
-
-

-
On the morning of March 23, the National Industrial Innovation Center of Precision Medicine was inaugurated in Chengdu Tianfu International Bio-town.2022-03-31View More
-
-
-
-
Cue Health on Tuesday afternoon announced a whopping year-over-year uptick in revenues for the fourth quarter, beating the consensus Wall Street estimate.2022-03-31View More
-
-
-

-
Liquid biopsy performed on serum samples taken from breast cancer patients can provide increasingly accurate information on cancer progression and enable earlier detection of cancer recurrence, a new study from the University of Eastern Finland and Kuopio University Hospital shows.2022-03-31View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

