
Home / News
-
-

-
collaboration, cardiovascular disease, blood test2026-01-09View More
-
-
-

-
US pharmaceutical group Merck is in talks to buy Revolution Medicines, a cancer drugmaker with a market capitalisation of more than $20bn in what would be the latest big deal in the red-hot biotechnology sector.2026-01-09View More
-
-
-

-
MSD (tradename of Merck & Co., Inc., Rahway, N.J., USA), today announced the successful completion of the cash tender offer, through a subsidiary, for all the outstanding shares of common stock of Cidara Therapeutics, Inc. (Nasdaq: CDTX) (¡°Cidara¡±).2026-01-08View More
-
-
-

-
SOPHiA GENETICS (NASDAQ: SOPH), a global leader in AI-driven precision medicine, and The University of Texas MD Anderson Cancer Center today announced a strategic collaboration that unites SOPHiA GENETICS' AI-powered analytics with MD Anderson's clinical and scientific expertise to accelerate data-driven cancer care through new tools that can accurately analyze, interpret and translate diagnostic results into clinical practice.2026-01-08View More
-
-
-

-
National Health Commission of the PRC recently convened a meeting in Beijing to outline ten practical initiatives for public service by 2026.2026-01-07View More
-
-
-

-
Adaptive Biotechnologies said last week that its new subsidiary Digital Biotechnologies has held an initial closing of its Series A preferred stock financing and expects to raise up to $15 million in the round.2026-01-07View More
-
-
-

-
Mdxhealth and the University of Oxford will test whether the firm's Genomic Prostate Score (GPS) assay can predict disease progression and treatment outcomes in prostate cancer patients, the company said Tuesday.2026-01-07View More
-
-
-

-
2. Current Conditions of Blood Coagulation Analyzers and Reagents in China As indicated by the release of the China Cardiovascular Health and Disease Report 2024, the current number of cardiovascular pat..2026-01-06View More
-
-
-

-
Original from: GlobeNewswire Burning Rock Biotech Limited (NASDAQ: BNR, the ¡°Company¡± or ¡°Burning Rock¡±), a company focusing on the application of next generation sequencing (NGS) technology in the f..2026-01-06View More
-
-
-

-
Corewell Health and Quest Diagnostics (NYSE: DGX) today announced the completion of the previously announced transaction to establish a laboratory services joint venture to expand access to innovative, quality and affordable laboratory services in Michigan.2026-01-06View More
-
-
-

-
Roche Diagnostics¡¯ VENTANA MMR RxDx Panel CDx Approved in China Roche Diagnostics¡¯ VENTANA MMR RxDx Panel companion diagnostic (CDx) reagent has officially obtained approval of NMPA. As ..2026-01-05View More
-
-
-

-
On 17 December 2025, the World Health Organization (WHO) announced the prequalification of two rapid antigen diagnostic tests (Ag-RDT) for SARS-CoV-2, the virus that causes COVID-19. The two tests are the SD Biosensor STANDARD Q COVID-19 Ag Test and the ACON Biotech Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing). This marks the first time that rapid antigen tests for SARS-CoV-2 have achieved WHO prequalification.2026-01-05View More
-
-
-

-
Sinopia Biosciences, Inc., a biotechnology company advancing novel therapeutics identified using its proprietary LEADS® drug discovery platform, announced today it has been awarded a research grant from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health (NIH), to further advance its data-driven drug discovery capabilities.2025-12-31View More
-
-
-

-
Biobeat Technologies, Ltd., developer of the first FDA-cleared, 24-hour ambulatory blood pressure monitoring (ABPM) system that is a patch-worn, cuff-less solution for diagnosis and treatment of hypertension, announced today the closing of a $50 million Series B equity financing.2025-12-31View More
-
-
-

-
Blood Coagulation Analyzer and Reagents 1. Overview of Domestic Blood Coagulation Market Conventional thrombosis and hemostasis diagnostic applications are mainly concentrated on screening for bleeding dis..2025-12-30View More
-
-
-

-
Italian in vitro diagnostics firm Diasorin said on Sunday that the US Food and Drug Administration has issued 510(k) clearance and CLIA-waived the firm's first assay for use on the Liaison Nes platform.2025-12-30View More
-
-
-

-
The EU's Unified Patent Court last week ruled in favor of Myriad Genetics in its patent dispute with South Korean firm GXD-Bio.2025-12-30View More
-
-
-

-
Applied BioCode said on Monday that is has submitted a new nucleic acid extraction claim for its BioCode Respiratory Pathogen Panel (RPP) to the US Food and Drug Administration, with the goal of expanding use of the panel by clinical laboratories.2025-12-30View More
-
-
-
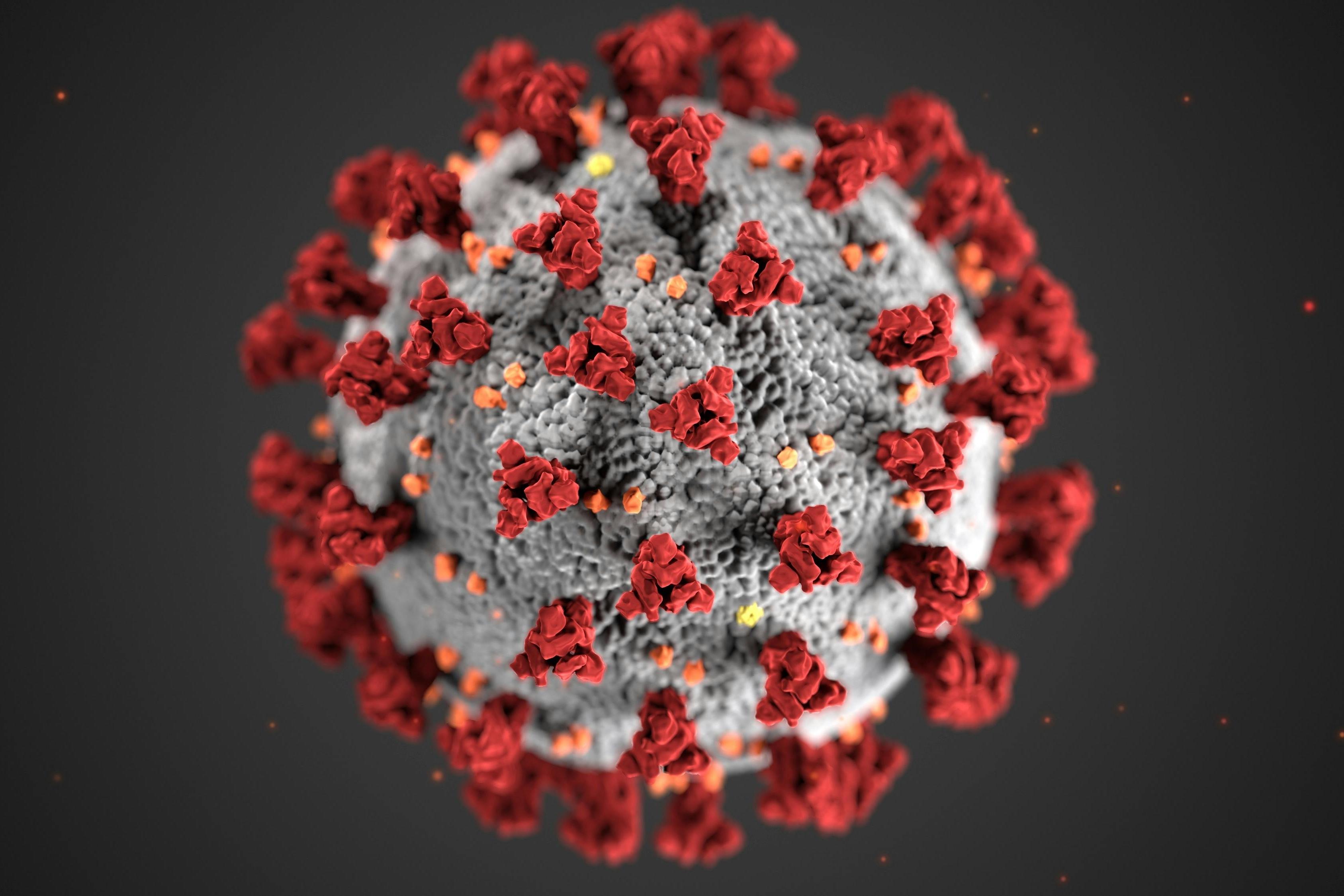
-
Following decades of cumulative efforts, a number of infectious disease tests and technologies broke new ground in 2025, hinting at rapid advances in the development of new systems and assays.2025-12-30View More
-
-
-

-
Chinese molecular diagnostics and precision medicine firm GenePlus Technology said on Sunday that it has filed for an initial public offering on the Hong Kong Stock Exchange.2025-12-30View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

