
Home / News
-
-

-
ELITechGroup is pleased to announce that they have obtained the IVDR certification for four new products including the brand new CMV RNA ELITe MGB Kit.2024-06-05View More
-
-
-
-
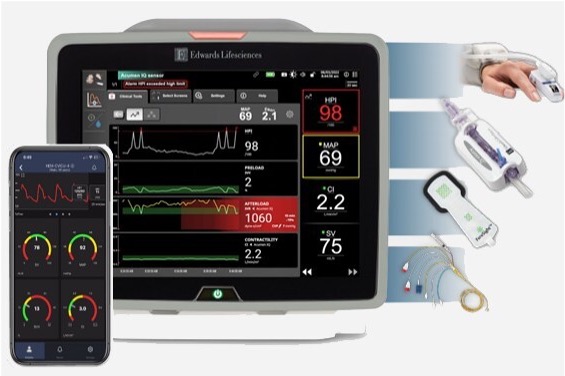
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, and Edwards Lifesciences (NYSE: EW), today announced a definitive agreement under which BD will acquire Edwards' Critical Care product group ("Critical Care"), a global leader in advanced monitoring solutions, for $4.2 billion in cash, unlocking new value creation opportunities and enhancing BD's portfolio of smart connected care solutions.2024-06-05View More
-
-
-

-
Maccura obtained the registration certificate of this product and was granted the invention patent in 2018. It is the first creatinine assay kit for anti-drug interference.2024-06-04View More
-
-
-

-
Watmind USA has been granted Emergency Use Authorization (EUA) by the US Food and Drug Administration (FDA) for its SpeedySwab Covid + FLU A&B Self-Test.2024-06-04View More
-
-
-

-
Illumina, Inc. (NASDAQ: ILMN) today announced that its Board of Directors has approved the spin-off of GRAIL. GRAIL is anticipated to spin off from Illumina on June 24, 2024, and has applied to list on Nasdaq as "GRAL."2024-06-04View More
-
-
-

-
Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.2024-06-03View More
-
-
-

-
Burning Rock Biotech Limited (NASDAQ: BNR and LSE: BNR, the ¡°Company¡± or ¡°Burning Rock¡±) has recently announced a collaboration with Bayer to develop next-generation sequencing (NGS)-based companion diagnostic assays (CDx), aiming to provide diagnostic methods, to enable treatment choice for patients with cancer in China, while driving innovation and development in cancer therapy. This collaboration will focus on the development of companion diagnostic products in China, jointly developing NGS-based CDx for Bayer's growing portfolio of precision cancer therapies.2024-06-03View More
-
-
-

-
QIAGEN (NYSE: QGEN; Frankfurt Prime Standard: QIA) and Myriad Genetics (NASDAQ: MYGN) today announced they will develop a globally distributable kit-based test for analyzing Homologous Recombination Deficiency (HRD) status. This next-generation sequencing (NGS) test aims to support research into personalized medicine in multiple solid tumor types, including ovarian cancer and is expected to enhance decentralized testing capacities once a regulated product is developed with pharmaceutical partners. The project builds on the recently announced master collaboration agreement between the two companies.2024-06-03View More
-
-
-

-
Oxford Nanopore Technologies has launched a new Pharmacogenomics (PGx) Beta Program with Twist Biosciences.2024-05-24View More
-
-
-

-
Point-of-care diagnostic firm Cue Health is laying off all of its employees and shuttering its operations.2024-05-24View More
-
-
-

-
T2 Biosystems announced Wednesday that it has regained compliance with a listing requirement to remain on the Nasdaq.2024-05-23View More
-
-
-

-
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today that the Tina-quant® lipoprotein Lp(a) RxDx assay has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) to identify patients who may benefit from innovative Lp(a)-lowering therapy currently in development. Lp(a) is emerging as an important, yet under-recognised, potential risk factor for cardiovascular disease, a major public health issue.2024-05-23View More
-
-
-

-
In the First Catalogue of Rare Diseases, which was jointly developed by the National Health Commission of the PRC and five other departments in 2018, there is a rare disease called IgG4-Related Disease (IgG4-RD).2024-05-22View More
-
-
-

-
Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced certification for its Guardant360® CDx blood test under the European Union¡¯s In Vitro Diagnostic Regulation (IVDR 2017/746). The certification from TÜV SÜD Product Service is for tumor mutation profiling in patients with any solid cancerous tumor and for companion diagnostic indications to identify patients who may benefit from certain targeted therapies for advanced non-small cell lung cancer and breast cancer.2024-05-22View More
-
-
-

-
The UK Medicines & Healthcare Products Regulatory Agency on Tuesday published a proposed framework for the recognition of in vitro diagnostics that have already been cleared in other jurisdictions.2024-05-22View More
-
-
-

-
With the application of the latex-enhanced immunoturbidimetry and the enzyme immunoassay amplification, the sensitivity of biochemical test items is greatly improved. Representative products applied in the biochemical technology platform in 2019-2020 are as follows:2024-05-21View More
-
-
-
-
The American Society of Clinical Oncology on Friday released guidelines to help guide use of multigene germline genetic testing panels for cancer patients.2024-05-21View More
-
-
-

-
Karius®, Inc., a leader in genomic diagnostics for infectious diseases, announced today that the Karius Test® has been granted designation as a Breakthrough Device from The Center for Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA), for use in the diagnosis and management of immunocompromised patients with suspected lung infections including lower respiratory infection and pneumonia.2024-05-21View More
-
-
-

-
Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.2024-05-20View More
-
-
-

-
OSF HealthCare, a 16-hospital integrated health system that serves patients in Illinois and Michigan, and DELFI Diagnostics, Inc., a developer of accessible blood-based tests that deliver a new way to enhance cancer detection, today announced a collaboration to utilize DELFI's FirstLook Lung blood-based cancer screening test to help improve screening rates. DELFI's collaboration with OSF was recently highlighted by the Biden Cancer Moonshot to expand accessibility to lung cancer screening.2024-05-20View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

