
Home / News
-
-

-
Thermo Fisher Scientific, the world leader in serving science, today introduced the Thermo Scientific™ Orbitrap Exploris™ EFOX Mass Detector, the industry¡¯s first high-resolution accurate mass (HRAM) Orbitrap system designed specifically for environmental and food safety laboratories. The new system addresses urgent global challenges around food and water quality testing in the face of persistent contaminants, such as per- and polyfluoroalkyl substances (PFAS), pesticides and other pollutants. With tailor-made workflows and some of the strongest targeted analysis technology in the field, laboratories can use the Orbitrap Exploris EFOX (Environmental and Food Organic Xenobiotics) to generate richer, compliant data faster in order to assess public health risks sooner.2025-10-28View More
-
-
-

-
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced a new self-collection solution for HPV testing in markets outside the United States. This new innovation simplifies at-home sample collection for patients and further automates lab processing using high-tech robotics with the BD COR™ System.2025-10-24View More
-
-
-

-
Original from: Roche ¡¤ Group sales grew by 7% at constant exchange rates (CER; 2% in CHF) in the first nine months, driven by high demand for our innovative medicines and diagnostics. ¡¤ P..2025-10-24View More
-
-
-

-
Thermo Fisher Scientific Inc. (NYSE: TMO), the world leader in serving science, today reported its financial results for the third quarter ended September 27, 2025.2025-10-23View More
-
-
-

-
Diasorin (FTSE MIB: DIA) today announced the signing of a supplier agreement with Quest Diagnostics (NYSE: DGX), a leading provider of diagnostic information services based in and serving the United States.2025-10-23View More
-
-
-

-
Quest Diagnostics Incorporated (NYSE: DGX), a leading provider of diagnostic information services, today announced financial results for the third quarter ended September 30, 2025.2025-10-22View More
-
-
-

-
Danaher reported a 5 percent year-over-year increase in third quarter revenues on Tuesday that was partially due to higher-than-anticipated revenues from Cepheid's respiratory testing business.2025-10-22View More
-
-
-

-
2.3 Development of Domestic Coagulation Enterprises With the development of the blood coagulation industry, more and more IVD companies have joined it and actively expanded the related services. • ..2025-10-21View More
-
-
-

-
Merck (NYSE: MRK), known as MSD outside of the United States and Canada, announced today the start of construction for a $3 billion, 400,000-square-foot pharmaceutical manufacturing facility at its Elkton, Virginia, site.2025-10-21View More
-
-
-

-
At the annual IDWeek meeting (https://idweek.org/), Bruker announced the U.S. Food and Drug Administration (FDA) clearance of Claim 7 and Claim 8 for its MALDI Biotyper® CA System, marking a significant advancement in clinical microbial identification capabilities. The clearance includes the MBT Compass HT CA software and MBT FAST™ Shuttle US IVD (Claim 7), as well as a major expansion of the FDA-cleared reference library (Claim 8), now encompassing 549 clinically validated microbial species across gram-positive and gram-negative bacteria, anaerobes and yeasts.2025-10-21View More
-
-
-

-
Mindray Officially Announces Plans to List in Hong Kong Mindray recently convened its 14th meeting of the 8th Board of Directors, which reviewed and approved the ¡°Proposal on Issuing H-Shares and Listin..2025-10-20View More
-
-
-

-
MVZ HPH Institute for Pathology and Hematopathology GmbH, today announced the availability of HPH MRD, a new tumor-informed circulating-tumor DNA (ctDNA) blood test for detecting minimal residual disease (MRD) in patients diagnosed with solid tumor cancers. The HPH MRD test is an in-house test manufactured by HPH that makes use of Haystack MRD technology and is available through a license from Haystack Oncology, a subsidiary of Quest Diagnostics® (NYSE: DGX), which developed and provides the Haystack MRD in-house developed test in the United States. The technology was purpose-built to detect ultralow levels of ctDNA with exceptional sensitivity and specificity, enabling the reliable identification of residual or recurrent disease.2025-10-20View More
-
-
-

-
Illumina, Inc. (NASDAQ: ILMN) today announced that GeneDx, a leader in genetic testing for rare diseases, is piloting Illumina's emerging constellation mapped read technology, evaluating its performance on regions of the genome that traditional short-read technologies historically have not resolved. GeneDx's early results illustrate the ability of constellation to rapidly identify hard-to-detect variants implicated in rare disease. GeneDx's Director of Laboratory Innovation Joe Devaney will present on the company's early experiences with the constellation technology today at the American Society for Human Genetics (ASHG) Annual Meeting in Boston.2025-10-20View More
-
-
-

-
In preliminary results reported on Wednesday after the close of the market, Becton Dickinson said its revenues in the fiscal fourth quarter of 2025 increased 8 percent year over year. The firm also announced the upcoming departure of its CFO and disclosed a transition plan for the position.2025-10-17View More
-
-
-

-
Samsung C&T (SCT), Samsung Electronics (SEC), and GRAIL, Inc. (Nasdaq: GRAL), today announced they have signed a binding Letter of Intent for a strategic collaboration to bring GRAIL's GalleriⓇ multi-cancer early detection (MCED) test to key Asian markets. SCT and SEC have also agreed to invest $110 million into GRAIL, a healthcare company whose mission is to detect cancer early when it can be cured, at a price of $70.05 per share of common stock.2025-10-17View More
-
-
-
-
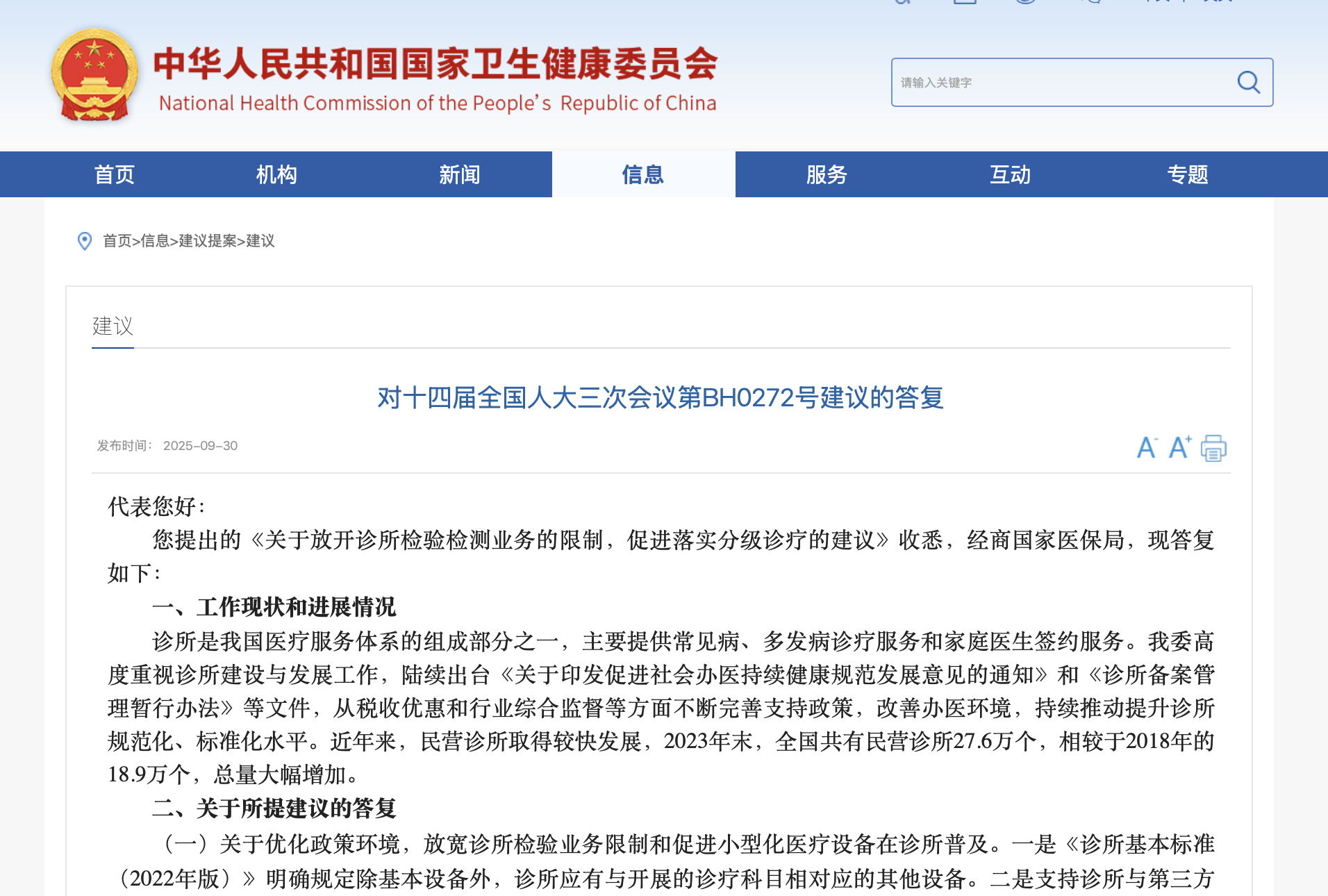
China¡¯s National Health Commission (NHC) recently responded to a deputies¡¯ proposal on ¡°Lifting Restrictions on Diagnostic Testing Services in Clinics to Promote the Implementation of Tiered Healthcare¡±: ..2025-10-16View More
-
-
-

-
Thermo Fisher Scientific, the world leader in serving science, has introduced the Applied Biosystems™ SwiftArrayStudio™ Microarray Analyzer, designed for fast and scalable sample analysis. In conjunction with two new arrays ¨C the Applied Biosystems Axiom™ PharmacoPro™ Array and the Applied Biosystems Axiom PangenomePro Array ¨C the analyzer combines four key genotyping processes into a single, integrated device, enabling researchers to cover the widest range of populations for Genome-Wide Association Studies (GWAS) and accelerate breakthrough discoveries in pharmacogenomics that will help drive the future of precision medicine forward.2025-10-16View More
-
-
-

-
Abbott (NYSE: ABT) today announced financial results for the third quarter ended Sept. 30, 2025.2025-10-16View More
-
-
-

-
OSR Holdings, Inc. (NASDAQ: OSRH) today announced that it has executed a definitive agreement to acquire Woori IO Co., Ltd. ("WORIO"), a pioneer in noninvasive glucose monitoring (NIGM), via a comprehensive share exchange. The acquisition reinforces OSRH's dedication to advancing biomedical innovation by adding a potential breakthrough technology for diabetes care, bringing truly needle-free glucose monitoring closer to patients globally and promising a safer, more convenient and accurate alternative to currently available technologies.2025-10-15View More
-
-
-
-
China leads the world in AI research publications related to the global medical device field over the past five years, according to a latest industrial white paper.2025-10-15View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

