
Home / News
-
-
-
Researchers have used laboratory-on-a-chip technology to create a low cost, sensitive tuberculosis (TB) test that could improve disease detection in high-endemic, under-resourced areas.2023-07-07View More
-
-
-

-
Tempus, a leader in artificial intelligence and precision medicine, today announced a new collaboration to develop a companion diagnostic (CDx) test with TScan Therapeutics, a clinical-stage biopharmaceutical company focused on the development of T cell receptor (TCR)-engineered T cell therapies (TCR-T) for the treatment of cancer patients.2023-07-07View More
-
-
-

-
Siemens and Alibaba Cloud signed a strategic cooperation agreement, in which both parties will leverage their technological advantages in their respective fields to jointly promote the integration of cloud computing, AI big models and industry, enabling Chinese enterprises to improve innovation and productivity, and injecting acceleration into the high-quality development of China's economy.2023-07-06View More
-
-
-

-
EDP Biotech and New Day Diagnostics announced on Wednesday that they will combine their assets to form a new company.2023-07-06View More
-
-
-

-
New research has uncovered protein signatures associated with different forms of Alzheimer's disease (AD), including markers found in plasma, cerebrospinal fluid (CSF), and brain tissue.2023-07-06View More
-
-
-

-
Recently, the Tianjin Medical Products Administration issued the "Notice on the Demonstration and Promotion of the Unique Identification of Medical Devices".2023-07-05View More
-
-
-
-
The US Food and Drug Administration (FDA) has granted approval for Lumos Diagnostics¡¯ FebriDx rapid point-of-care (POC) test.2023-07-05View More
-
-
-

-
Today, in collaboration with single cell genomics company, Honeycomb Biotechnologies (¡°Honeycomb¡±), Revvity (NYSE: RVTY) announced the launch of a suite of new solutions and services that expand the frontiers of single cell biology: the HIVE™ CLX Single-Cell RNAseq Solution, BeeNetPLUS analysis workflow, and for single cell researchers based in the United States, a new HIVE CLX Service offering.2023-07-05View More
-
-
-
-
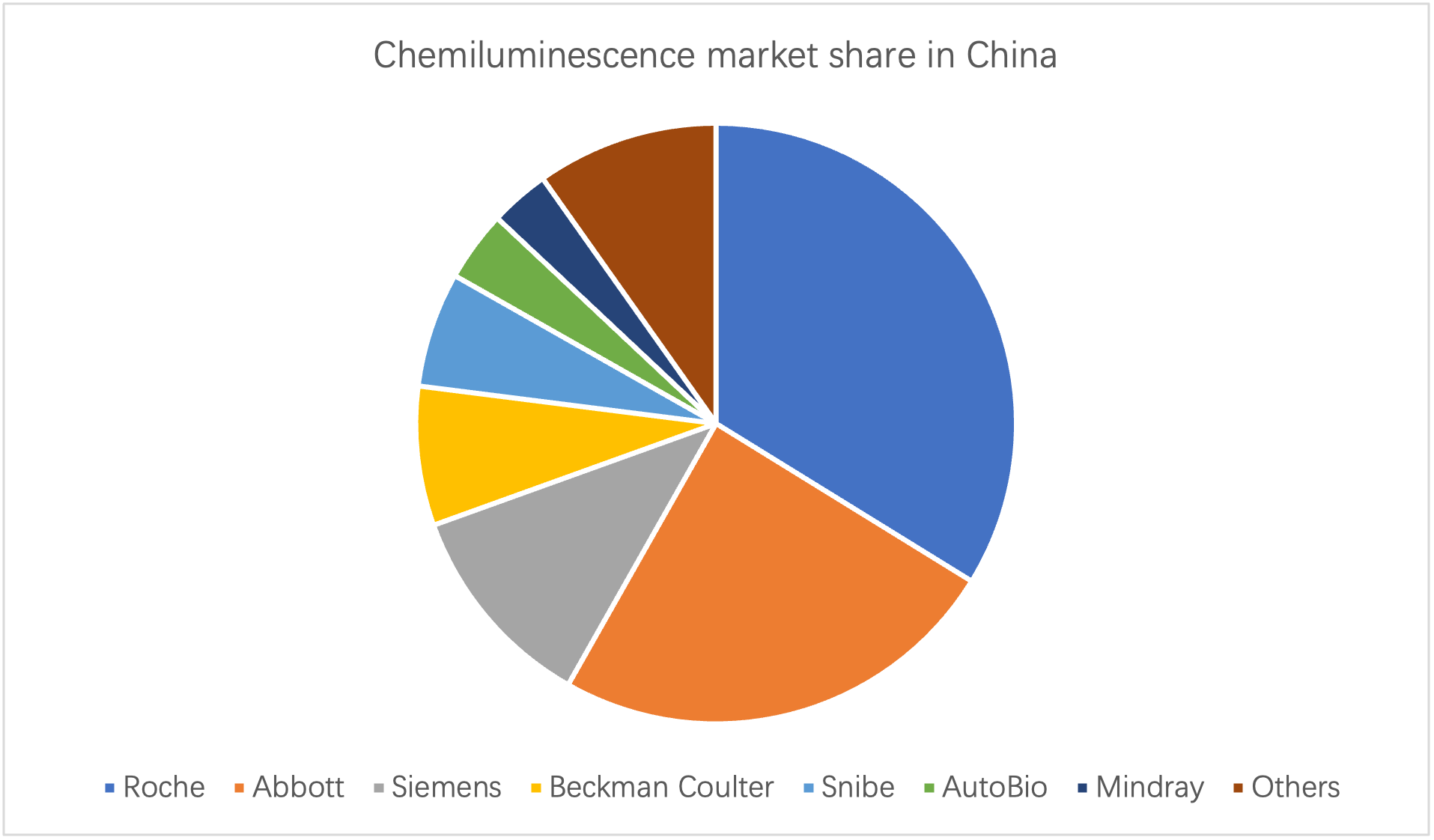
In recent years, the market scale of immunodiagnostic reagents in China has been increasing year by year. In 2020, the market size of China's immunodiagnostic reagent industry reached 32.1 billion yuan, an increase of 24.42% year-on-year.2023-07-04View More
-
-
-

-
Renalytix has received De Novo marketing authorisation from the US Food and Drug Administration (FDA) for its KidneyIntelX.dkd prognostic test.2023-07-04View More
-
-
-

-
Houston-based single-cell analysis company CellChorus announced on Wednesday that it has been awarded a $2.2 million Small Business Innovation Research (SBIR) Fast-Track grant from the National Institute of General Medical Sciences (NIGMS).2023-07-04View More
-
-
-

-
On June 29, Merck announced an investment of approximately 70 million euros to expand its Nantong Life Science Center in the heart of the Yangtze River Delta region.2023-07-03View More
-
-
-

-
LumiraDx Limited (Nasdaq: LMDX), a leading innovator in diagnostic technologies, today announced the submission of its first 510(k) application to the U.S. Food and Drug Administration (FDA) for the clearance of its ground-breaking 5-minute COVID Ultra Test. LumiraDx submitted a traditional 510(k) Dual Submission for LumiraDx SARS-CoV-2 Ag Ultra with CLIA Waiver for the commercial distribution at point of care of the LumiraDx Platform, including the LumiraDx Instrument, with the LumiraDx SARS-CoV-2 Antigen (Ag) Ultra test, and the LumiraDx SARS-CoV-2 Ag Quality Control Swab Kit.2023-07-03View More
-
-
-

-
Helio Genomics said Thursday that its sister company, Laboratory for Advanced Medicine & Health Group (LAMH), has received approval from the National Medical Products Administration (NMPA) in China for its cell-free DNA-based liver cancer detection test. The assay is the first of its kind to receive this approval.2023-07-03View More
-
-
-

-
At 9:30 a.m. on June 30, Adicon was officially listed on the Hong Kong Stock Exchange. Adicon Holdings (09860.HK) is offering 33,192,500 shares globally, of which 3,320,000 shares are offered in Hong Kong, China and 29,872,500 shares are offered internationally, with a 15% over-allotment option.2023-06-30View More
-
-
-

-
Eli Lilly and Company (NYSE: LLY) and Sigilon Therapeutics, Inc. (Nasdaq: SGTX) today announced a definitive agreement for Lilly to acquire Sigilon, a biopharmaceutical company that seeks to develop functional cures for patients with a broad range of acute and chronic diseases.2023-06-30View More
-
-
-

-
ARUP Laboratories today announced that the U.S. Food and Drug Administration (FDA) has approved AAV5 DetectCDx™ as a companion diagnostic to aid in the selection of adult patients eligible for treatment with ROCTAVIAN™ (valoctocogene roxaparvovec-rvox).2023-06-30View More
-
-
-

-
Based on the advantages of their respective fields, the two companies will carry out in-depth cooperation in life information and support, in vitro diagnosis, medical imaging, overall solutions for two cancer screening, hospital department equipment, information construction, etc., and jointly establish the "Hybribio-Mindray Standardized Laboratory" to achieve complementary advantages and resource sharing.2023-06-29View More
-
-
-

-
Bio-Techne announced on Thursday that it is acquiring Swiss spatial biology firm Lunaphore for an undisclosed amount.2023-06-29View More
-
-
-

-
PamGene International said Tuesday that the European Innovation Council's Accelerator program will provide €7.5 million ($8.2 million) in funding to develop and market the firm's prognostic test for immunotherapy response in metastatic cancer treatment.2023-06-29View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

