
Home / News
-
-
-
In some areas with weak medical resources in China, it is difficult for the public to obtain quality medical services and have to seek treatment across provinces and regions, which is a heavy economic and time burden.2023-06-28View More
-
-
-

-
MOROCCO ¨C Techniques Science Sant¨¦ (T2S), a distributor of high-tech medical equipment based in Casablanca, has expanded a multi-year agreement with American company GE Healthcare, with a focus on ultra-modern modern technology.2023-06-28View More
-
-
-

-
Thermo Fisher Scientific Inc., the world leader in serving science, has completed its acquisition of MarqMetrix, a privately held developer of Raman-based spectroscopy solutions for in-line measurement. The terms of the deal were not disclosed.2023-06-28View More
-
-
-

-
Roche said Tuesday that it secured US Food and Drug Administration 510(k) clearances for a pair of assays to aid diagnosis of Alzheimer's disease measuring levels of beta-amyloid and tau proteins.2023-06-28View More
-
-
-
-
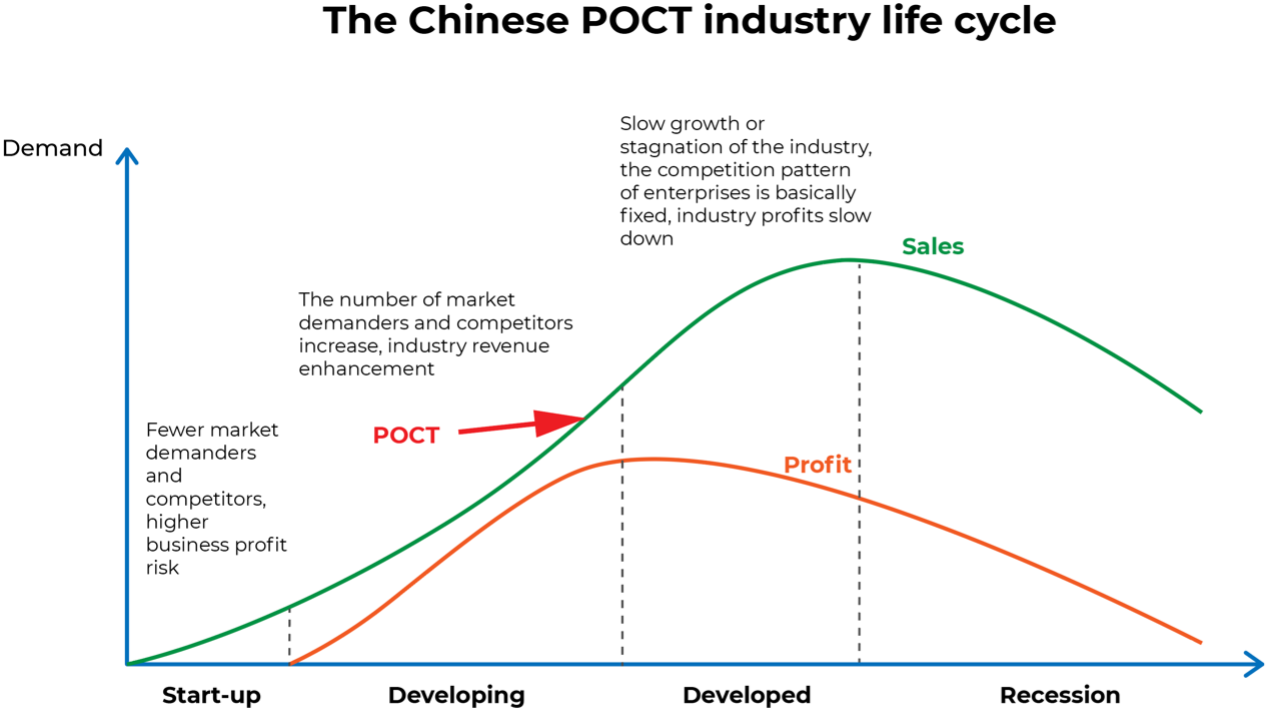
POCT industry started late in China, the overall market size is still small currently, and the penetration rate in hospitals and other terminal sites is low, so there is a huge potential development space. Qianzhan Institute predicts that from 2022-2027, the Chinese POCT market will rise by about 9% CAGR to about 18 billion yuan in 2027.2023-06-27View More
-
-
-

-
Prenetics Global Limited, a leading genomics-driven health sciences company, and Prof. Dennis Lo today announce they have entered into an agreement to establish a joint venture named Insighta. The board of directors of Prenetics Global Limited has unanimously approved the transaction.2023-06-27View More
-
-
-

-
Oxford Nanopore Technologies (Oxford Nanopore) and leading China-based diagnostic companies, Zheijiang Digena Diagnostic Technology Co. Ltd (Digena) and Wuhan Dgensee Clinical Laboratory Co., Ltd (Dgensee), today announced a series of strategic collaborations that aim to bring nanopore sequencing technology and instruments to the clinical market in China.2023-06-27View More
-
-
-
-
As the world's most active emerging market, the influence of China's market on the entire global scientific instrument market is rising year by year, and revenue in China has become the most important part of the global performance of many multinational companies.2023-06-26View More
-
-
-

-
Rice University bioengineers said this week that they have demonstrated a low-cost, point-of-care DNA test for HPV infections that could make cervical cancer screening more accessible in low- and middle-income countries where the disease kills more than 300,000 women each year.2023-06-26View More
-
-
-

-
Quest Diagnostics said it completed its previously announced acquisition of Haystack Oncology.2023-06-26View More
-
-
-

-
Recently, the Chinese Center for Disease Control and Prevention (CDC) issued a Notice on the public consultation of 2 standards.2023-06-21View More
-
-
-

-
Paige is enhancing its suite of AI-enabled solutions designed to support pathologists in the diagnosis of breast cancer while decreasing subjectivity and the tedium of manual analysis.2023-06-21View More
-
-
-

-
The US Food and Drug Administration announced on Tuesday that it has launched a voluntary pilot program intended to reduce the risks of laboratory-developed tests used to select patients for oncology therapies.2023-06-21View More
-
-
-
-
On March 30 2023, Kalorama Information indicates that the global IVD market is now over $124 billion, with operations in more than 20 key healthcare and medical areas, such as infectious disease immunoassays, POC, molecular microbiology, clinical chemistry, etc.2023-06-20View More
-
-
-

-
Inex Innovate of Singapore plans to launch a PCR-based endometrial cancer detection test this summer, following interim results from a clinical study with researchers at the Chinese University of Hong Kong that were presented here at the Association of Molecular Pathology 2023 Europe Congress this week.2023-06-20View More
-
-
-

-
China-based, blood cancer early detection and monitoring technology company, SeekIn has released new research on its multi-cancer early detection (MCED) test, OncoSeek.2023-06-20View More
-
-
-

-
Recently, Genalive, a joint venture between BGI Almanahil Health for Medical Services (a wholly owned subsidiary of BGI Genomics) and Tibbiyah Holding (a wholly owned subsidiary of Saudi Al Faisaliah Group), opened its independent clinical laboratory.2023-06-19View More
-
-
-

-
Qiagen on Thursday announced a systematic review and publication of the clinical relevance of test values from its QuantiFeron-TB Gold Plus TB1 and TB2 blood collection tubes.2023-06-19View More
-
-
-

-
Breast Cancer Canada said on Thursday that it has launched a new grant program in collaboration with AstraZeneca Canada and Illumina.2023-06-19View More
-
-
-

-
On June 15, 2023, the inaugural meeting of Institute of Bioinformatics of Shanghai Academy of Experimental Medicine (hereinafter referred to as "Institute of Bioinformatics") and Gene Sequencing Bioinformatics Research Summit Forum was held in Intercontinental Hotel in Jing'an, Shanghai. Ltd.2023-06-16View More
-

- CAIVD WeChat
Subscription Account

- CAIVD WeChat
Channels
China Association of In-vitro Diagnostics
Part of the information in our website is from the internet.
If by any chance it violates your rights, please contact us.

