



1. Status Quo and Development of Biochemical Analysis Instruments
1.1 Overview
A biochemistry analyzer is an instrument that automatically completes the steps of sampling, adding reagents, removing interfering substances, mixing, constant temperature reaction, automatic monitoring, data processing, post-cleaning, etc. It can provide the clinical testing of biochemistry, hematology, and immunology items in the hospitals at all levels, including the clinical biochemical parameters for liver function, kidney function, blood lipid, diabetes, infection, rheumatism, immunity, etc., thereby providing an important scientific basis for the disease prevention, diagnosis, and treatment. The application of biochemistry analyzers has greatly improved the accuracy and precision and working efficiency of biochemical tests, and meets the requirements for the laboratory medicine. Presently, it has become one of the essential instruments for clinical diagnosis in medical institutions.
In recent years, the global market of biochemistry analyzers developed steadily, with a compound growth rate of approximately 4.30% from 2015 to 2020. The global market size of biochemistry analyzers reached 3.679 billion US dollars in 2019 and 3.790 billion US dollars in 2020. The Chinese market of biochemistry analyzers grew rapidly, with a compound growth rate of approximately 5.73% from 2015 to 2020. In 2019, the Chinese market size of biochemistry analyzers reached 582 million US dollars. Biochemistry analyzers include semi-automatic biochemistry analyzers and fully automatic biochemistry analyzers. Among them, the fully automatic biochemistry analyzers have a large market share, accounting for nearly 78.4% of total market in 2019. The biochemistry analyzer market is diversified; the top three enterprises, Roche, Danaher, and Hitachi, occupy nearly 50% of the total market share, they have possessed the key and core technologies and patents, and their products have been extensively recognized in the markets. The import and export trade and downstream industries will affect the needs for the biochemistry analyzers.
The Chinese biochemistry analyzers have experienced the technical progress from manual, semi-automatic to fully automatic biochemistry analyzers. A number of Chinese enterprises have produced fully automatic biochemistry analyzers. However, the domestic biochemistry analyzers started late, and the technology was weak. Presently, domestic companies represented by Mindray, KHB, and Dirui have successfully produced biochemistry analyzers that can compete with foreign companies. In terms of the market conditions, large hospitals and large laboratories prefer the fully automatic biochemistry analyzers of foreign brands because of high requirements on the technical parameters, performance, and brand of the equipment. The small- and medium-sized hospitals usually use small- and medium-sized biochemistry analyzers because of small sample size and cost consideration. Thus, the domestic high-end market is still dominated by foreign brands, and with the foreign brands entering the low-end markets, the competition in the markets of small- and medium-sized hospitals will become fiercer.
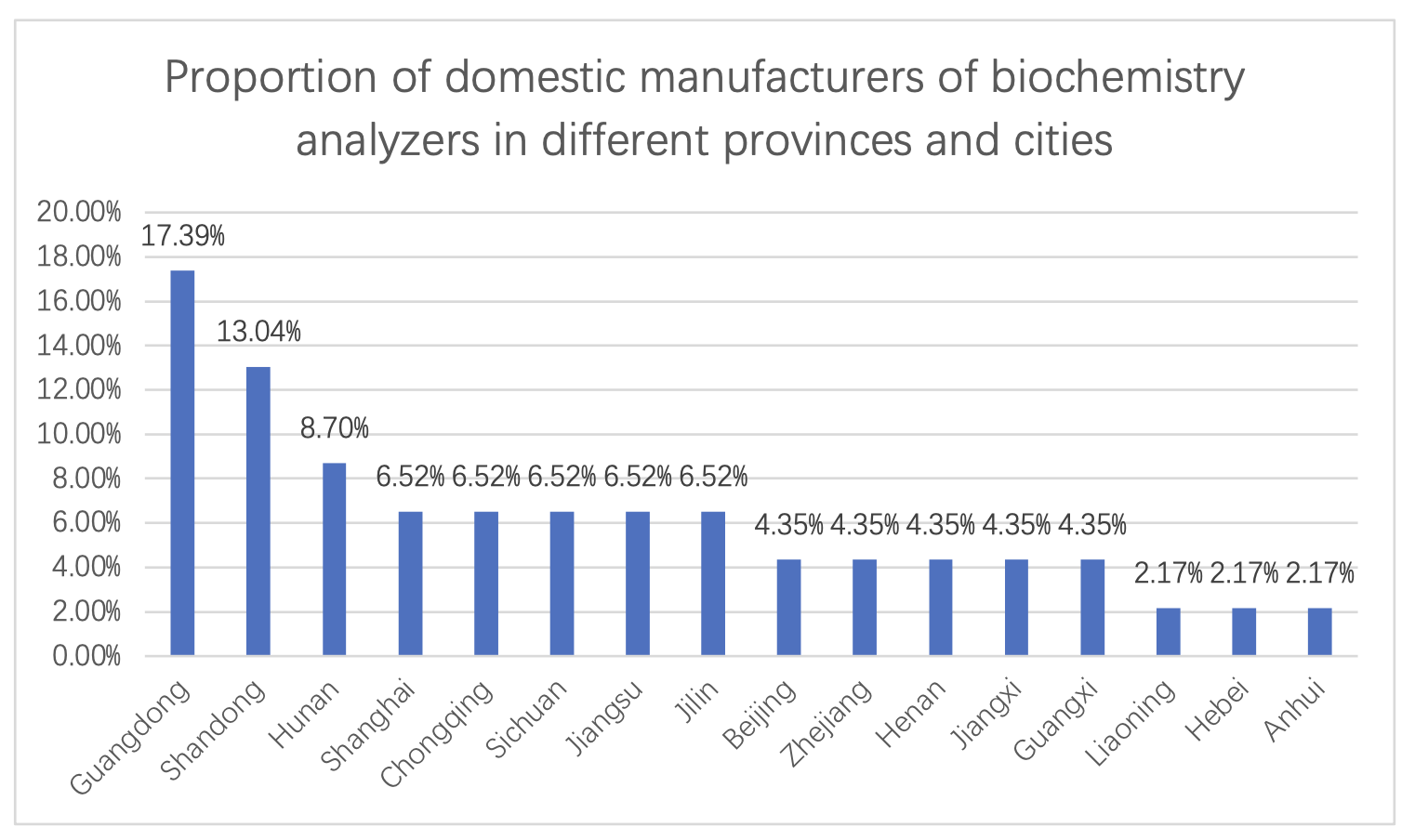
Based on the data of CFDA, the above figure shows that there are 90 new registration certificates for the production of fully biochemical analysis instruments in 2019-2020, covering 46 domestic manufacturers and 234 models of instruments. These manufacturers are mainly distributed in Guangdong, Shandong, Hunan, Shanghai, Chongqing, Sichuan, Jiangsu, Jilin, etc. Geographically, these manufacturers of biochemistry analyzers are mainly located in the eastern and southern coastal areas and the central area. The main market participants of domestic biochemistry analyzers are Mindray, Dirui, Medicalsystem, KHB, ZY Biotech, etc.
Last: In Vitro Diagnostic Industry in China - Clinical Chemistry I
Next: In Vitro Diagnostic Industry in China - Clinical Chemistry III